1
/
of
2
Vitalograph
Vitalograph Asma-1 Respiratory Monitor
Vitalograph Asma-1 Respiratory Monitor
Regular price
$174.97 AUD
Regular price
Sale price
$174.97 AUD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Vitalograph Asma-1 Respiratory Monitor
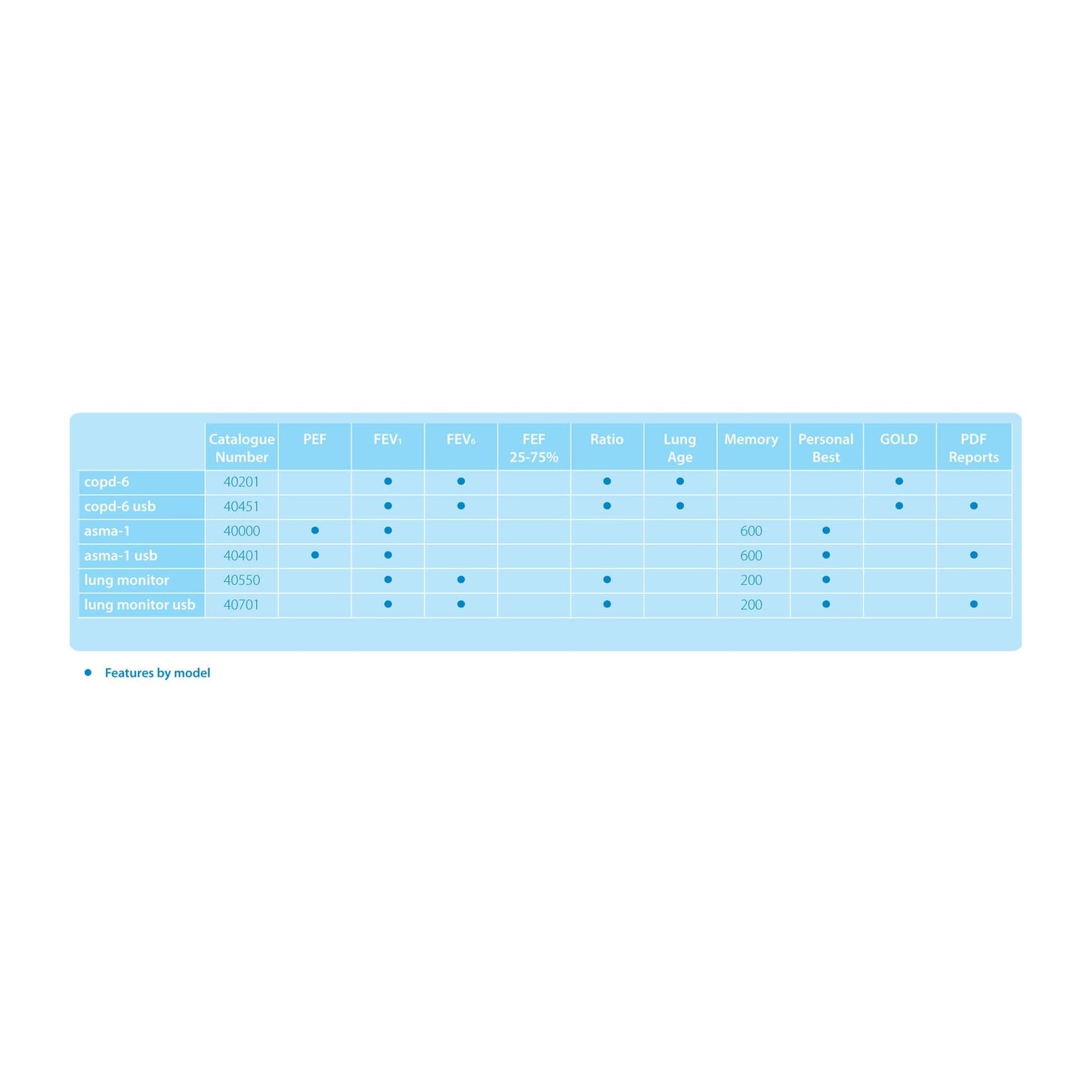
The Vitalograph asma-1 is a simple home use electronic respiratory monitor that measures PEF and FEV1 and may be integrated with smart phone or tablet for ePRO data collection. It offers greater accuracy than mechanical peak flow meters and eliminates the need for paper record cards.
- Measures PEF and FEV1
- FEV1 has less diurnal variability and is less effort dependent than PEF
- Displays % of personal best for both PEF and FEV1
- PEF and FEV1 zones can be personalised
- Automatically assesses test quality
- Automatically stores best values
- Large, easy to read display
- Stores 600 test sessions
- Suitable for use in clinic with Vitalograph Bacterial Viral Filters (eco BVF)
- Uses long life AAA batteries
- Language independent
- Child version measures PEF, FEV0.5, FEV0.75 and FEV1
| Product | Vitalograph asma-1 |
| Model Number | 4000 |
| Part Number | 40000, 40400, 40300, 40050 |
| Description | Vitalograph asma-1, Vitalograph asma-1 USB, Vitalograph asma-1 Bluetooth, Vitalograph asma-1 Serial |
| Size | 113 x 63 x 48mm |
| Weight | 55g net |
| Parameters Displayed (Adult version) | PEF & FEV1 |
| Parameters Displayed (Child version) | PEF, FEV0.5, FEV0.75 and FEV1 |
| Operating Temperature Range | 17 - 37°C |
| Measuring Principle | Stator/rotor mass flow meter |
| Flow / Volume Range | PEF: 25 - 840 L/min BTPS FEV1: 0 - 9.99 L BTPS |
| Accuracy | FEV1 better than +/-5% or +/-100ml (whichever is greater) PEF better than +/-10% or +/-25L/min (whichever is greater) |
| Repeatability | Better than 5% or 10L/min, whichever is greater |
| Cleaning | Alcohol wipe, especially the mouthpiece, weekly |
| Disposal Methods | Recycle |
| Performance standard | ISO 23747:2007; ATS/ERS 2005 |
| Safety Standards | IEC 60601-1:2005 |
| Medical Safety Standards | Medical Devices Directive 2007/47/EC; ISO 13485:2003 |
| Designed and manufactured under | CMDR FDA 21CFR820 |
Share